The Food and Drug Administration recently granted full approval of Pfizer's COVID-19 vaccine which now leads to the question, what's the difference between this and the emergency use authorization the agency earlier allowed?
This means an AP News report said, the shot of Pfizer for individuals 16 years old and above, has now gone through the same rigorous trial and regulatory assessments as the other long-established inoculations available.
COVID-19 vaccines in the United States were at first, rolled out under the EUA of the FDA, which allows the latter-mentioned, to speed the medical products' availability in times of public health emergencies.
Under the process, the agency excluded some of its regular requirements for data. It also reduced its processes for the vaccines become injectable several months earlier than would have been possible during normal situations.
ALSO READ: 'Worrying' Mutation Detected in the UK
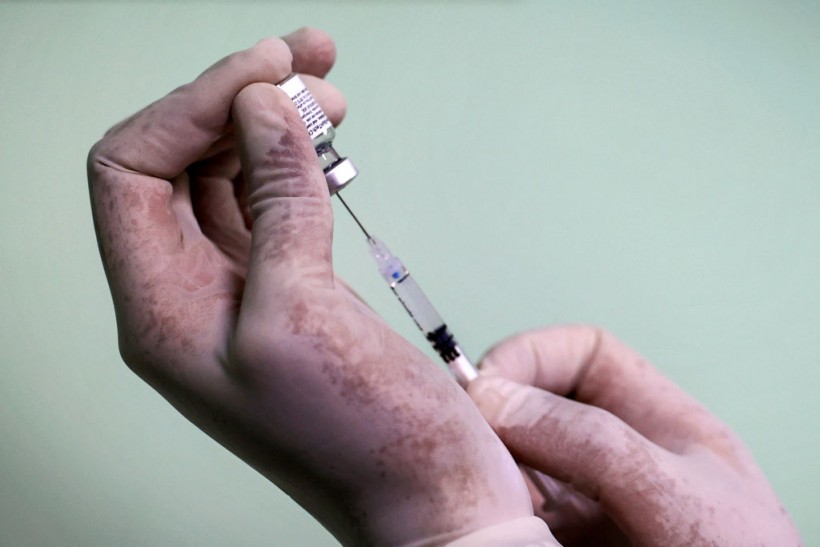
A medic prepares a dose of the Pfizer-BioNTech COVID-19 coronavirus vaccine at a medical center in Gaza City on August 23, 2021.
Six-Month Follow-Up Data Required
The vaccine of Pfizer, along with injections from Moderna and Johnson & Johnson, still went through testing in tens of thousands of individuals in order to establish safety and efficacy from the COVID-19 virus.
Initially, the FDA required the vaccine manufacturers to submit around only two months of safety monitoring data on study volunteers, the period when side effects are most likely to take place.
Meanwhile, for full approval, it is a requirement from the FDA for the companies to submit six months of follow-up data.
Inspectors from the agency also visited the plants where vaccines are developed and assessed every step of the production process to be extra guaranteed that the injections are made under safe and sterile conditions.
Since vaccines are usually given to otherwise healthy people, they are commonly subject to more regulatory scrutiny compared to other medical products, which include prescription medicines.
Pfizer, Moderna, and Johnson & Johnson
Now with full approval from the FDA, Pfizer is now carrying the strongest endorsement of the FDA in terms of safety and efficacy.
Public health experts are hoping the change will convince more people who have not yet been vaccinated to get their shot and spur more businesses to require their employees to get their vaccines.
Moderna has also sought the FDA's full approval, while Johnson & Johnson has announced it is hoping to seek application as well, later this year.
The vaccine of Pfizer is still available for individuals aged 12 to 15 years old under EUA. The full vaccine, on the other hand, is not applicable to boosters. The FDA has yet to decide independently if an extra shot is necessary for healthy individuals.
EUA vs Full Approval
Before the use of a vaccine in the US, it needs to be thoroughly reviewed by the FDA. Specifically, the agency assesses the assesses every vaccine's efficacy, safety, potency, and purity as demonstrated through laborious testing, which typically starts with animal testing.
If animals are experiencing the intended impact of vaccination minus substantial side effects, then the tests will carry on in human trials which are performed in three phases.
If the vaccine continues exhibiting promise, testing will move ahead to Phase 3, where thousands of participants will be assessed. Again, researchers here try to understand the real efficacy and safety of the vaccines and later on, identify if there are side effects.
Upon approval of the vaccine, safety monitoring will carry on through the use of surveillance tools such as the Vaccine Adverse Event Reporting System or VAERS, the Vaccine Safety Datalink, and the Biologics Effectiveness and Safety System.
According to an Oregon Vaccine News report, Pfizer, Moderna, and Johnson & Johnson vaccines have all been given an EUA from the FDA. Such authorizations may be granted only during a public health emergency and when there are not sufficient, approved, and existing substitutes.
While different from the FDA approval, it does not mean that such vaccines have not undergone rigorous safety testing, as well. Just the same as the process for FDA approval, vaccine research for FDA is conducted through three phases.
Related report on the full approval of COVID-19 vaccine by the FDA is shown on NBC News's YouTube video below:
RELATED ARTICLE: COVID-19 Delta Variant Spreads Fast; More Travelers From Abroad Want Another Vaccine Booster Shot in the US
Check out more news and information on COVID-19 and Vaccines in Science Times.









!['Cosmic Glitch' in Einstein's Theory of General Relativity Could Be Explained in This New Scientific Tweak [Study]](https://1721181113.rsc.cdn77.org/data/thumbs/full/53435/258/146/50/40/cosmic-glitch-in-einsteins-theory-of-general-relativity-could-be-explained-in-this-new-scientific-tweak-study.jpeg)



