To acknowledge further early mammalian development, a group of researchers from the California Institute of Technology partnered through one teammate from the Francis Crick Institute and yet another from the University of Cambridge in the United Kingdom. This researcher discloses employing several different types of stem cells to produce mouse embryos in an article published in the scientific journal Nature Cell Biology.
The preceding study has demonstrated that, when mammalian embryos progress, they diversify into several kinds of cell bodies. Scientists have, however, discovered that stem cells play an involvement in the processes although the mechanisms involved in this are unexplained.
The research conducted three types of stem cells to create a mouse embryo that grew to the point where it possessed a heart beating and the foundations of a brain throughout this latest analysis, according to the published work in Nature.
Creation of Artificial Embryos
To create such embryos, the experts primarily analyzed communication amongst stem cell divisions in normally growing mouse embryos. They learned to identify the components of such messages as well as how they were carried out.
They "deciphered the code" and then extracted three distinct kinds of stem cells that comprised the cellular masses in earliest embryological development: pluripotent, which later developed to form muscle cells, and two other varieties that grew to produce the amniotic sac and placenta. They also recorded the numbers within each category of stem cells.
The following stage was to endeavor to produce a mouse embryo from zero in a lab environment utilizing the three different types of stem cells. The scientists created and sustained one embryo that progressed enough already to allow them to observe its growth.
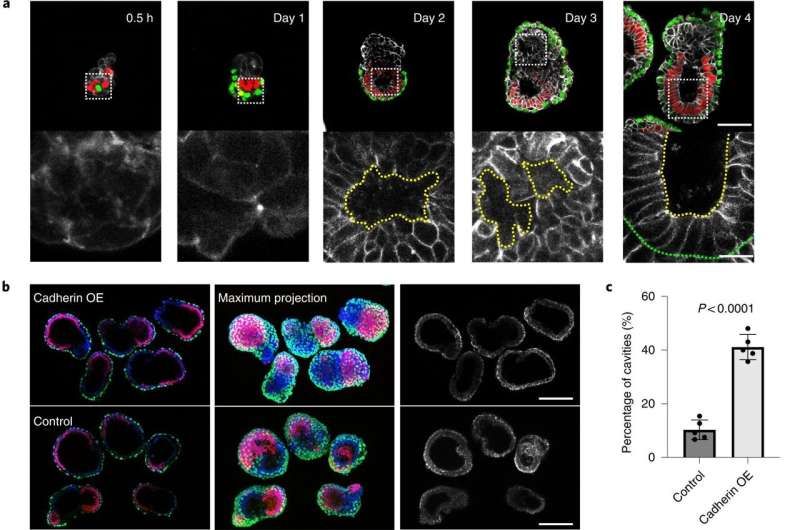
Correct self-organization is necessary for proper morphogenesis. a, Time course of the assembly of ETX embryos stained to reveal E-cadherin (monochrome), Oct4 (red) and Gata4 (green). The bottom row of images are magnifications of the images above and show E-cadherin staining around a nascent cavity, as indicated by the dashed yellow lines. The dashed green line indicates the boundary between the ES and XEN compartment. Scale bar, 5 μm. b, Representative images showing Oct4 (red), Gata4 (green), E-cadherin (monochrome) and DAPI (gray) staining in day 4 cadherin OE ETX structures formed by combining E-cadherin OE ES cells with P-cadherin OE TS cells and wild-type XEN cells. ETX structures formed by combining wild-type cells were used as a control. Scale bars, 100 μm. c, Comparison and quantification of joined cavity formation in cadherin OE and control ETX structures. n = 361 (control group) and n = 253 (cadherin OE group). N = 5 for each condition. The data are presented as means ± s.d. Statistical significance was determined by unpaired two-tailed Student’s t-test. d, Representative image showing Oct4 (red), Gata4 (green), laminin (monochrome) and DAPI (blue) staining in day 4 cadherin OE ETX structures formed by combining E-cadherin OE ES cells with P-cadherin OE TS cells and wild-type XEN cells. ETX structures formed by combining wild-type cells were used as a control. Scale bars, 100 μm. e, Quantification of the structures that contained continuous or discontinuous laminin. n = 40 ETX structures per condition. N = 3. The data are presented as means ± s.d. Statistical significance was determined by unpaired two-tailed Student’s t-test. f, Self-organization principles in stem cell-derived ETX embryos. Differential expression of E-, K- and P-cadherins enables the sorting of ES (epiblast-like), XEN (VE-like) and TS (TE-like) stem cells. Wild-type ES cells with low E-cadherin expression and wild-type TS cells with low P-cadherin expression exhibited detrimental global sorting efficiency. This could be overcome by overexpressing E-cadherin in ES cells and P-cadherin in TS cells to increase the efficiency of ETX embryo formation. Proper morphogenesis, including cavity formation, basement membrane formation (purple) and symmetry breaking can only be observed in well-sorted structures.
ALSO READ: Lab Mouse Embryo Grown from Stem Cells Features a Nervous System and Heart Beat
Mammalian Embryonic Maturation
As Phys.org reported, to investigate how everything affected embryo maturation, the authors repeated the experiment with genetically manipulated cells. They determined that they could reproduce a portion of the development of the brain abnormalities observed in human fetuses. They believe their research might help to understand what happens when mice (or humans) miscarry.
Because mammalian eggs contain some of the tiniest in the natural world, they are difficult to control in experiments. The human zygote, for example, is just 100 m in diameter, scarcely visible to the naked eye, and only one-thousandth the content of a Xenopus egg. Furthermore, because human zygotes are not generated in quantities equivalent to sea cucumber or frog zygotes, sufficient material for biochemical research is difficult to collect. A female usually ovulates less than 10 eggs at a time. Mammalian embryos are developed within another creature rather than in an external environment as a final barrier. It was only later that some of these interior circumstances could be replicated and proliferation observed in vitro, according to a study from the National Library of Medicine.
Despite these challenges, understanding mammalian cleavage was worth the wait, since it turned out to be notably distinct from the majority of processes of embryonic cell division. The mammalian oocyte is liberated from the ovary and therefore is whisked into the oviduct by the fimbriae. Fertilization takes place in the oviduct's ampulla, which is near the ovary. Meiosis is finished at this point, and the first cleavage occurs around a day later. Cleavages within mammalian eggs include the longest in the animal world, occurring between 12 and 24 hours. Meanwhile, cilia in the oviduct urge the embryo into the uterus, where the first cleavages occur, as the NLM report added.
RELATED ARTICLE: World's First Synthetic Embryo Grows Without Sperm, Egg Cell, and Uterus
Check out more news and information on Biology in Science Times.

![Extinct Giant Salmon Had Tusk-Like Spikes Protruding Out of Its Snout That Can Easily Kill Shark, Other Large Marine Animals [Study]](https://1721181113.rsc.cdn77.org/data/thumbs/full/53304/89/56/50/40/extinct-giant-salmon-had-tusk-like-spikes-protruding-out-of-its-snout-that-can-easily-kill-shark-other-large-marine-animals-study.png)