Advancements in nanotechnology have led to the development of a unique nanomaterial that can effectively fight both Gram-positive and Gram-negative bacteria. This breakthrough revolutionizes antibacterial treatments and can potentially solve the challenges of antibiotic resistance.
Infections From Bacterial Species
Healthcare-associated infections and the rise in multi-drug resistant bacteria are some issues often encountered in wound care. According to the Centers for Disease Control and Prevention, antibiotic resistance has become an urgent global public health threat that has killed at least 1.27 million people worldwide and is associated with almost 5 million deaths in 2019. While most bacterial species in question are widespread, they can cause more serious, untreatable infections in immunocompromised individuals.
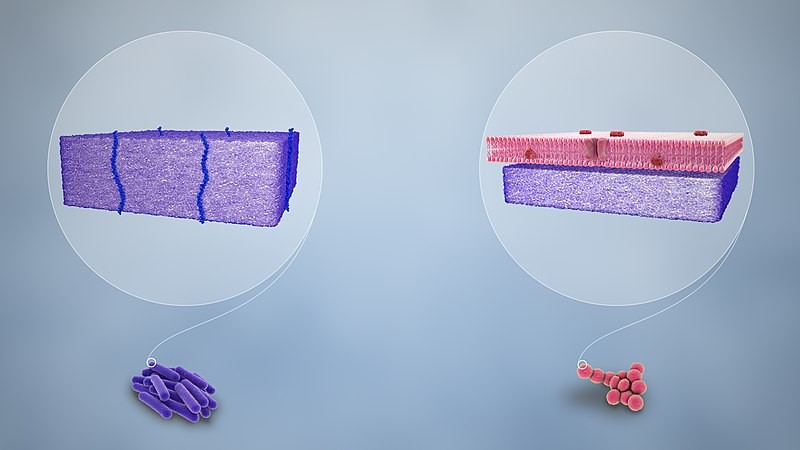
Bacterial species are identified using Gram staining to determine whether they are Gram-positive or Gram-negative. These bacteria differ in terms of their outer membrane structures and composition.
Gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus, possess a bacterial membrane mainly composed of peptidoglycans. On the other hand, Gram-negative bacteria, such as Pseudomonas aeruginosa, have inner and outer bacterial membranes made of phospholipids with a thin peptidoglycan layer. The latter is also a healthcare-associated bacterium with problematic resistance to broadband antibiotics.
READ ALSO: Lipid Nanoparticles From Combined Treatments Show Potential in Killing Antibiotic-Resistant Bacteria
Light-Controlled Bioactivity
To fight bacterial infections effectively and selectively, a team of researchers has developed a bactericidal nanomaterial that can be directed against Gram-positive or Gram-negative bacteria. This innovation offers a new approach to treating healthcare-associated infections without relying on antibiotics.
Led by Mrinmoy De from the Indian Institute of Science in Bengaluru, India, scientists have created a UV-visible-light-responsive nanomaterial. They believe that strain-selective bactericidal activity must be achieved for better treatment.
The researchers designed a functionalized nanomaterial made of molybdenum disulfide to achieve a bactericidal agent that can selectively interact with the chemical surfaces of both bacterial strains. It also contains azobenzene moieties attached to positively charged quaternary amino groups.
Molybdenum disulfide is a bactericide, while the quaternary amino groups allow membrane depolarization. On the other hand, the azobenzene moieties introduce a light-driven switch in the nanomaterials from an elongated trans into a curved cis form to allow selective surface interactions.
De used different chemical probes and optical measurements and his colleagues to determine the effectiveness of both the cis and trans forms in killing bacteria. They found that the transform of the nanomaterial depolarized and thoroughly pierced the bacterial membrane of Gram-negative Pseudomonas aeruginosa. This enabled the molybdenum disulfide nanomaterial to generate intracellular reactive oxygen species and kill the bacteria. In contrast, the Gram-positive methicillin-resistant Staphylococcus aureus responded to the cis form more effectively. Its cell wall was damaged and ruptured by specific interactions.
The nanomaterial has demonstrated efficacy after successfully healing methicillin-resistant Staphylococcus aureus-infected wounds in mice models. The wounds closed entirely after ten days of treatment with the cis reagent, while the common conventional antibiotic treatment with vancomycin did not heal quickly.
By flipping the UV switch from the trans ground state to the cis form, it becomes possible to control each type of bacteria selectively. According to the researchers, the effectiveness of their nanomaterial against methicillin-resistant Staphylococcus aureus can also be extended to other selective bacterial infections.
RELATED ARTICLE: Novel Molecule Can Attack Hard-to-Kill Gram-Negative Bacteria
Check out more news and information on Bacteria in Science Times.














