The United States Food and Drug Administration will decide in the coming week if it is authorizing the first-ever COVID-19 vaccines for children aged five years old and below.
Fox 5 New York reported that the COVID-19 vaccine for kids five and younger could start as early as the week of June 20, the White House said recently.
Up to 20 million U.S. children under 5 could be eligible for a COVID-19 vaccine starting the week of June 20. https://t.co/wb3XElZFbY
— FOX 10 Phoenix (@FOX10Phoenix) June 9, 2022
If the FDA and the Centers for Disease Control and prevention approve, it would mean almost every American is eligible for a vaccine.
The administration announced it has 10 million vaccine doses for states, territories, tribes, pharmacies, community health centers, and others to pre-order. Additional doses will be shipped in the weeks ahead.
ALSO READ: Volunteer for Pfizer's Experimental COVID-19 Vaccine Shares Side Effects from Trial
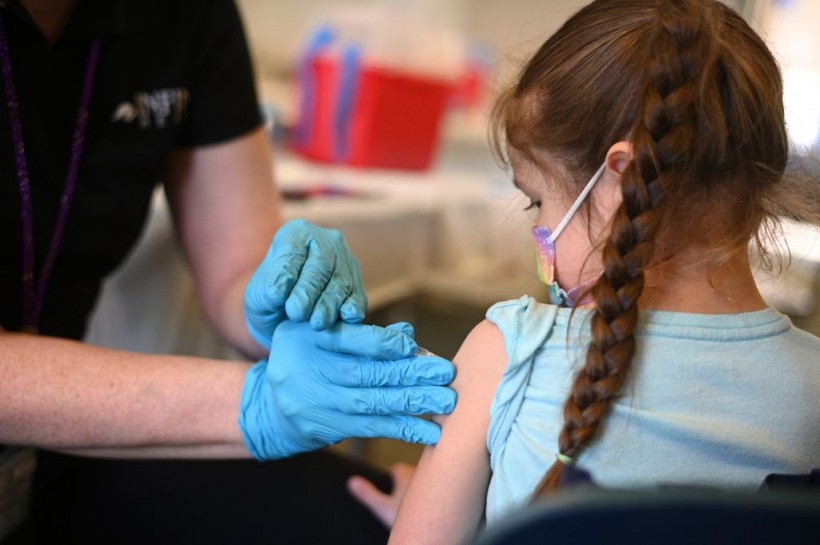
A nurse administers a pediatric dose of the Covid-19 vaccine to a girl at an L.A. Care Health Plan vaccination clinic at Los Angeles Mission College in the Sylmar neighborhood in Los Angeles, California, on January 19, 2022.
20 Million Young Children to Get Vaccinated
A report from the White House stated that 85 percent of young children below five years old will live within "five miles of potential vaccination site."
In the news release, the White House also said the vaccination program of the administration for the youngest children of America would focus on meeting the specific needs of this age group and their families, recognizing many parents and guardians will choose to get their children vaccinated through their primary care doctor or pediatrician.
As specified in a similar MPR News has asked FDA to authorize three doses of its COVID-19 vaccine for children aged six months to four years old. Every dose is one-tenth of the amount adults are given.
Moderna has asked FDA to authorize two shots for children aged six months to five years old, each containing one-quarter of the dose adults receive.
Young children are the last group of Americans who have not been recommended to be given a COVID-19 vaccine. Up to approximately 20 million US children below five years old would become eligible for vaccination if the government permits one or both shots, although it is not clear how popular shots will be.
Pfizer and Moderna Vaccines
A CBS News report said the window for jurisdictions to preorder the first wave of jabs, two million doses of vaccine of Moderna and two million doses of Pfizer, closed on Wednesday. In total, pharmacies were able to request up to a threshold of half a million doses from each brand.
The first wave of shots was not ordered in equal amounts. Specifically, vaccinators ordered 58 percent of Pfizer's total threshold was preordered, while only 34 percent of Moderna's was requested.
According to the officials, some states and jurisdictions had only ordered Moderna, while others ordered only Pfizer.
Meanwhile, the CDC had warned local officials that providers needed to place orders in the first wave unless they could receive the store shipments "arriving on Juneteenth holiday," the same report said.
An official from the administration said they, at this point, don't have numbers that can determine one way or the other, and their job is to make both brands available in equal numbers.
A report about the COVID-19 vaccine for children under five years old is shown on TODAY's YouTube video below:
RELATED ARTICLE: COVID-19 Vaccine for Children: Pfizer Anticipates Filing for FDA Complete Approval to Vaccinate Kids Aged 2-11
Check out more news and information on COVID-19 and Vaccines in Science Times.