The US Food and Drug Administration (FDA) announced on Wednesday, November 30, its approval of the use of feces-based microbial treatment for the prevention of recurring life-threatening diarrhea.
Dr. Peter Marks, the director of FDA's Center for Biologics Evaluation and Research, said that recurrent Clostridioides difficile infection (CDI) impacts an individual's quality of life and can be life-threatening. The first FDA-approved fecal microbiota product represents an important milestone as it provides an additional approved option to prevent the infection.
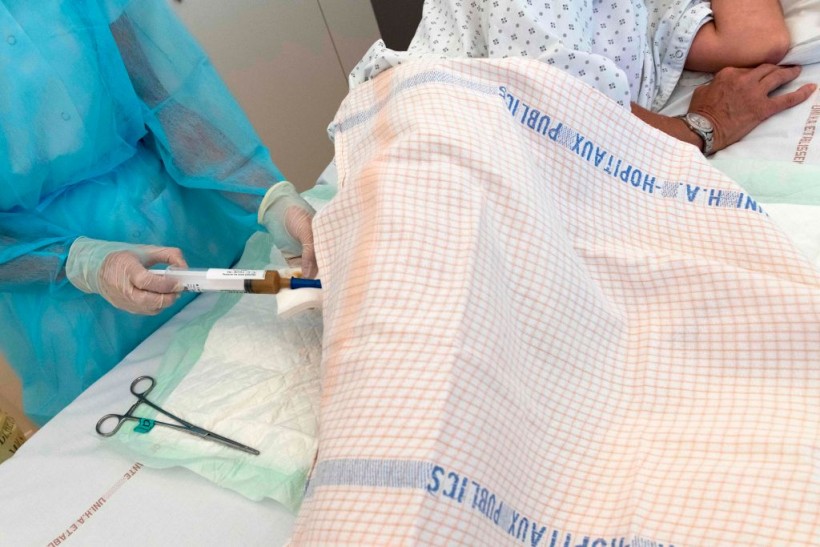
A patient is injected with a syringe filled with stool to treat patients with serious infections of the colon by Fecal microbiota transplantation (FMT), also known as Gut Flora Transplant (GFT), at the Centre Hospitalier Universitaire (CHU) in Clermont-Ferrand, central France on July 26, 2019.
Feces-Based Treatment is Years in the Making
The approval was many years in the making, as per ARS Technica. Researchers worked for many years to harness the protective qualities of the gut microbiota in healthy people's intestines and stool. Early research proved that fecal matter is useful for restoring balance and blocking infection in those whose microbiomes are disturbed, which can be due to disease from using antibiotics.
Doctors pushed informally in trying an array of methods to transplant fecal microbiota from healthy people to the guts of patients with problems. They used enemas or tubes through the nose and oral poop-packed capsules to transplant healthy microbiota from donors.
This method is known as fecal microbiota transplant (FMT) that has been used to treat ailments, including irritable bowel syndrome, with varied success. According to Johns Hopkins Medicine, FMT can control the recurrence of C. diff infection, and it can be performed in children and adults.
C. diff bacteria can cause diarrhea and inflammation in the colon in which severe forms could be life-threatening. People with dysbiosis or problems in the microbiota have C. diff in their intestines that produce toxins, leading to organ failure. Older people and immunocompromised people are usually hospitalized due to the infection.
The pressing need for effective treatment against C. diff forces regulators to evaluate the regulating and standardizing of something as unruly and myriad as fecal matter. But after many years of microbial sleuthing, synthetic slurries, stool donations, and clinical trials, the FDA finally gave its approval to the first feces-based treatment product.
READ ALSO: Fecal Transplant Cures Belgian Man With Auto-Brewery Syndrome
First Feces-Based Treatment
Rebyota is the first feces-based treatment the US FDA approved to prevent recurrent CDI. It is a blend of donor stool, saline, and laxative solution that is given in a single treatment. As ARS Technica reported, it contains a concentration of 10,000,000 live organisms per milliliter.
Switzerland-based Ferring Pharmaceuticals screens stool donors to ensure they have a healthy microbiota. In Phase II clinical trial with 262 participants published last month, scientists reported having high prevention rate than the placebo group with a rate of 70.6% and 57.5% respectively.
They considered the product to be successful in preventing recurring CDI if after eight weeks C. diff then not return following the treatment. The clinical trial also showed no serious side effects from the treatment.
Ferring president Per Falk said in a press release that the approval is a major breakthrough in harnessing the power of the human microbiome to address unmet medical needs, especially for people living with C. diff.
RELATED ARTICLE: Fecal Transplant Pills a Potential Food Allergy Treatment for People Allergic to Peanuts Based on Clinical Trial Results
Check out more news and information on Medicine and Health in Science Times.














