Scientists discovered a new pathway for the formation of sulfur particles in Venus' atmosphere. Using new computer techniques, researchers have developed a new understanding of the likely operation of Venus' complicated atmosphere. Following the application of sophisticated computational modeling, a group of scientists now propose a new route for the formation of disulfur within Venus' clouds.
New Sulfur Pathway Could Solve Mysterious UV Absorber on Venus Atmosphere
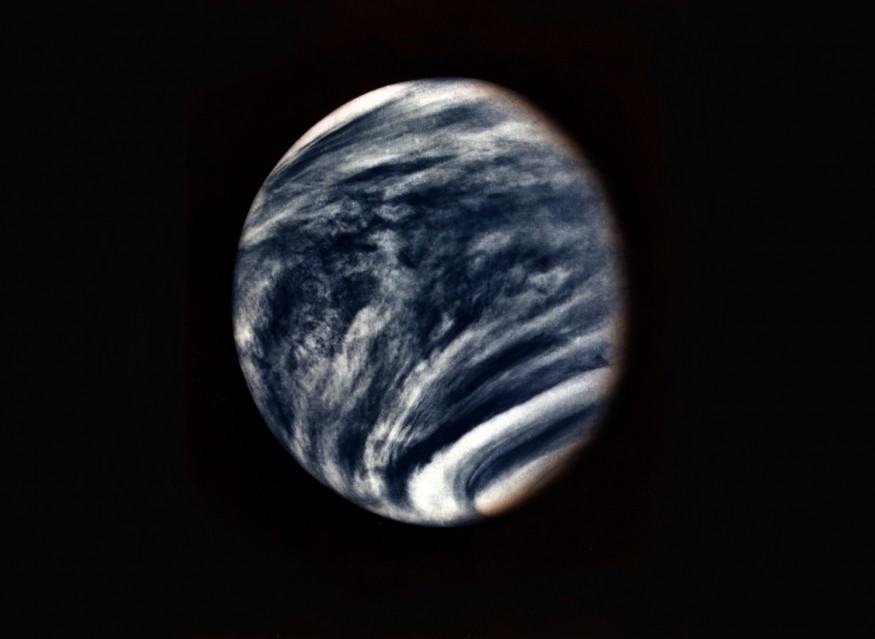
Venus, according to Space.com, is obscured by thick sulfuric acid clouds. It is the brightest object in the sky after the sun and moon because the clouds reflect most of the sunlight that falls on the planet. However, spacecraft and ground-based observations have revealed the presence of a previously unknown ultraviolet light absorber in the atmosphere.
The unknown absorber in Venus's clouds absorbs solar energy effectively at ultraviolet (UV) and blue wavelengths, according to Geophysical Research Letters, but its vertical location, either above or below the top cloud level (about 70 km altitude), is unknown.
The new research may aid in solving the long-unsolved mystery of Venus's mysterious ultraviolet absorber by identifying a new pathway for the formation of sulfur particles.
Computational Chemistry Technique Essential in Studying the Sulfur in Venus' Atmosphere
One of the paper's authors, James Lyons, a senior scientist at the Planetary Science Institute, said that for the first time, they are using computational chemistry techniques to determine which reactions are most important in sulfur formation, rather than waiting for laboratory measurements or using highly inaccurate estimates of the rate of unstudied reactions.
Computational methods are useful because it can be challenging and dangerous to work with the chemicals and compounds found in the Venusian atmosphere, such as sulfur, chlorine, and oxygen.
ALSO READ : Life on Venus Not True, New Study Debunks Long-Held Belief of Astronomers Saying There's Signs of Life
Formation of Sulfur in Venus' Atmosphere
Lyons stated that the atmosphere of Venus contains a lot of sulfur dioxide (SO2) and sulfuric acid particles, and we can expect sulfur particles to be produced by the ultraviolet destruction of SO2. They progress from atomic S (sulfur) to S2, S4, and finally S8. However, he was curious about how this process begins and how S2 forms.
S2 could be formed by the reaction of two sulfur atoms. S2 and S2 molecules can then combine to form S4, and so on. Condensed sulfur particles can form from S8 condensation or the condensation of S2, S4, and other allotropes, which then rearrange to form condensed S8.
Lyons stated that sulfur particles, as well as yellow sulfur, are primarily composed of S8, which has a ring structure. S8 is more resistant to UV light destruction due to its ring structure than the other allotropes.
To make S8, we can start with two S atoms and make S2, or we can use another pathway, as the researchers did in their paper. According to the researchers, they discovered a new S2 formation pathway involving the reaction of sulfur monoxide (SO) and disulfur monoxide (S2O).
The researchers believe that combining separate sulfur atoms is a much faster way to form disulfur than breaking down sulfur dioxide (SO2) by sunlight to form sulfur monoxide (SO) and disulfur monoxide (S2O).
The research was published in Nature Communications.
RELATED ARTICLE: Is Life Possible on Venus? Scientists Investigate Habitability on the Hottest Planet in the Solar System
Check out more news and information on Space in Science Times.
© 2025 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.











