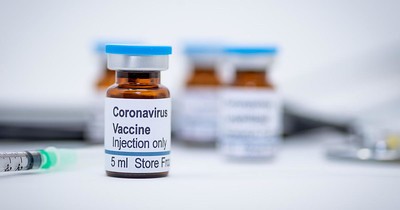
As the new coronavirus (COVID-19) spreads, the numbers globally, increase in terms of deaths, tested positive, and seen signs and symptoms. Because of this government agencies, universities and biotech companies scramble - some independently, others in collaboration-for a vaccine to have the virus contained.
Some companies have shared progress in their development of the coronavirus vaccine, and some, through genetic-based vaccines, has already delivered samples to various health agencies for examining.
Despite these initiatives, the approval process for this type of vaccine is much more demanding compared to most medicines. And, even if the samples sent to pass the clinical evaluations without any problem, the production of ready-to-use coronavirus vaccine will perhaps, take 12 to 18.
The reason for this is that because vaccines are administered to healthy people as prevention. Stanford University microbiology and immunology professor and senior fellow at the Freeman Spogli Institute for International Studies, Dr. David Relman elaborated, certainly, no one would want healthy people to get sick.
What the Vaccines help the Immune System
An individual's immune system keeps him well by identifying the difference between harmful and healthy cells. More so, a virus doesn't have the ability to reproduce on its own or by itself. It infects or contaminates a host sell, goes to the nucleus and captures the genetic material of the cell, forcing it to produce more viruses. When damaging pathogens occupy and begin to reproduce, the immune system identifies through their shapes.
Immune systems are imperfect. In some patients, their response to infections comes with an overreaction causing the inflammation in the lungs, a specific condition also called the acute respiratory distress syndrome. This has been detected in patients with flu and coronavirus.
One gets vaccinated through the injection of safe variation of a pathogen. This then simulates an infection. For instance, an individual doesn't get sick although some recipients of the vaccine may experience a minor symptom such as mild fever.
However, his white blood cells generate antibodies to fight the said pathogen as if it is a real and serious threat.
Why does it take months?
The Food and Drug Administration (FDA) licenses and approves a vaccine before it gets used for the public. Additionally, vaccines are made in laboratories and examined on animals before they go for clinical trials with humans. Dr. Martinez emphasized clinical tests are focused on safety.
As for vaccines, she added, they "are given to healthy adults before the actual patients receive them."
Relatively, tests are done in phases. In pre-clinical exams, the vaccine is tried in animals to gauge toxicity, the efficiency of the dosage, and the methods for administering it which include injection, oral, intranasal, and more.
One of the experts knowledgeable about a study on vaccines said, if the vaccine can protect animals as shown in the lab tests, it can be purified enough, to be tested on a human being.
© 2026 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.












