A key challenge in limiting the transmission of severe acute respiratory syndrome coronavirus 2, or SARS-CoV-2, is determining infected individuals.
Despite substantial and stunning advances in vaccine technology, a Medical Dialogue specified that the COVID-19 global pandemic is not over yet.
Now, researchers from Japan have created a new ant-body method for the rapid and dependable detection of SARS-CoV-2 that does not need a blood sample.
Essentially, the ineffective identification of SARS-CoV-2-infected people has severely restricted the worldwide response to the COVID-19 pandemic, and the high asymptomatic infection rates between 16 and 38 percent have exacerbated this condition.
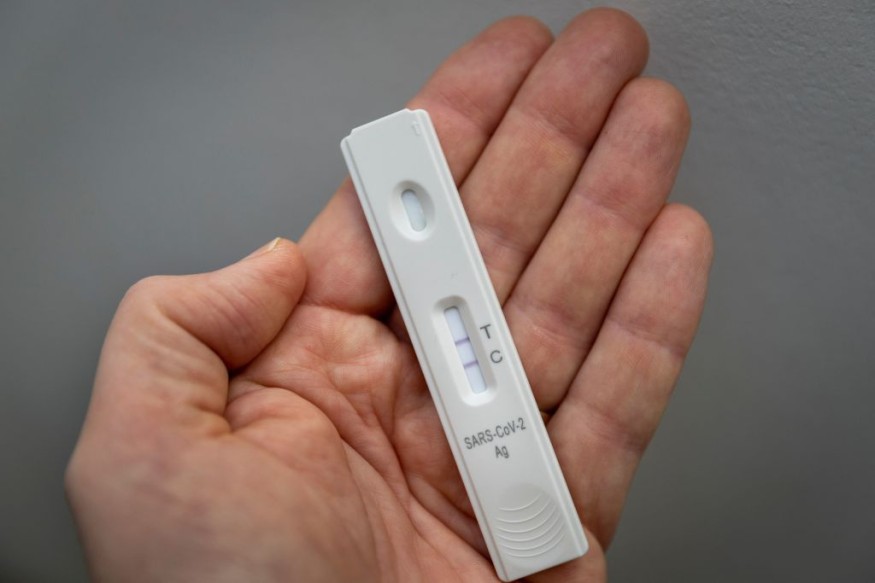
Detecting SARS-CoV-2-Specific Antibodies
The prevailing detection approach gathers samples by swabbing the throat and nose. Nonetheless, the application of this approach is limited by its long detection time of four to six hours, high cost, and requirement for specialized equipment and medical personnel, specifically in resource-limited nations.
A substitute and complementary approach for the confirmation of COVID-19 infection involves the detection of SARS-CoV-2-specific antibodies.
Testing strips based on nanoparticles are present globally for point-of-care testing in many nations.
They specifically generate sensitive and dependable results within 10 to 20 minutes, although they need blood samples collected through a finger prick using a lancing device.
This approach is painful and increases the danger of infection or cross-contamination, and the used kit components suggest a plausible biohazard risk.
Interstitial Fluid Tested
According to the study's lead author, Leilei Bao, from the Institute of Industrial Science, The University of Tokyo, to develop a minimally invasive detection assay that would prevent these drawbacks, they explored the notion of sampling and examining the interstitial fluid or ISF, which can be found in the human skin's epidermis and dermis layers.
Even though the antibody levels in the ISF are roughly 15 to 25 percent of those in blood, it was still possible that anti-SARS-CoV-2 IgM/IgG antibodies could be identified and that ISF could function as a direct substitute for blood sampling.
After showing that ISF could be appropriate for detecting antibodies, the authors developed an innovative technique to sample and test the ISF.
Senior author Beomjoon Kim explained that first, they developed biodegradable porous microneedles made of polylactic acid that's drawing up the ISF from human skin, a similar Outbreak News Today report said.
New antibody detection method for coronavirus that does not require a blood sample @SciReports #ScientificReports #coronavirus #SARS-CoV-2 #COVID-19 #bloodsample #COVIDinfection #infectiousdiseases #antibody #pandemic https://t.co/p3BurZh2qq
— Medical Dialogues (@medicaldialogs) July 3, 2022
Then, he added, they developed a paper-based immunoassay biosensor "for the detection of SARS-CoV-2-specific antibodies."
Detection within 3 Minutes
Through the integration of the said two elements, the researchers developed a compact patch capable of on-site detection of the antibodies within three minutes.
Such a novel detection device has great possibility for the rapid screening of coronavirus and many other infectious diseases that is safe and acceptable to patients.
Lastly, this approach holds promise for use in many nations regardless of their economic state, which is a key aim for the worldwide management of infectious diseases.
Related information about COVID-19 and Antibodies is shown on Beckman Coulter Dx's YouTube video below:
RELATED ARTICLE : Mouthwash Can Eliminate COVID-19 in 30 Seconds in the Lab, Scientists Continue Testing Its Efficacy in Patients
Check out more news and information on COVID-19 in Science Times.
© 2026 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.












