In a world where the oil and gas infrastructure and facilities that we rely on face constant degradation, Sreekar Parimi, an expert mechanical engineer, stands as a beacon, championing the integration of cutting-edge corrosion mitigation technologies to ensure longevity and safety.
Today, the design and engineering industry is undergoing a radical transformation. With the increasing complexity of infrastructures, facilities, and projects, the demand for solutions that offer durability, safety, and resilience has skyrocketed. As industries expand and technology advances, the challenges presented by the environment, from corrosion to wear and tear, are more pronounced than ever. The essence of modern engineering is not just about creating; it's about preserving, maintaining, and prolonging the life of what's been built. This is where corrosion engineering and mitigation come into play, bridging the gap between innovation and sustainability.
Sreekar Parimi stands out as a paragon in this evolving landscape. With over a decade of experience in mechanical engineering and a deep understanding of materials, he has carved a niche for himself, particularly in the complex and crucial field of corrosion mitigation. His journey has seen him tackle some of the most challenging environments, from packages to large facilities in the Oil and Gas and Energy industries, where the stakes are high and the margin for error is slim. What sets Sreekar apart is not just his profound knowledge but his ability to apply it innovatively. He has been responsible for classifying equipment based on corrosion risks and defining methodologies for regular evaluations, ensuring efficient allocation of resources without compromising safety.
His career is adorned with achievements that speak volumes about his expertise. From successfully designing an early production facility in Egypt with a production capacity of 180MMSCFD of gas, identifying potential pitfalls in material selection, to steering major projects away from looming corrosion disasters, his proactive approach has saved millions and ensured safety. Another testament to his adept problem-solving was evident when he tackled galvanic corrosion issues in a facility, introducing a simplified and effective classification system that ensured the right materials were used in the right places.
But beyond his technical achievements, Sreekar Parimi is also a thought leader and an active member of the corrosion community. His affiliation with NACE (now renamed AMPP), active participation in renowned conferences, and constant engagement with industry experts showcase his commitment to staying at the forefront of corrosion engineering innovations. His dedication to learning is not self-contained. He generously shares his insights, training fellow engineers, and has even penned standards that encapsulate years of industry experience.
We had the privilege to delve deeper into the world of Sreekar Parimi, discussing his extensive experience in deploying corrosion mitigation strategies. Through our conversation, it became clear that for Sreekar, engineering is not just a job; it's a passion, a mission to ensure that the infrastructures and facilities of today stand the test of time, weathering every challenge thrown their way. His innovative approach, combined with a deep understanding of materials and their interactions with the environment, has solidified his position as a stalwart in the design and engineering of the oil and gas upstream industry.
In the dynamic arena of mechanical engineering, where every decision can have profound implications, Sreekar Parimi exemplifies the blend of expertise, innovation, and dedication needed to navigate the future of oil and gas facilities.
We're excited to learn more about your craft, Sreekar. Can you tell us about a time when you had to deploy a corrosion mitigation strategy for a building or facility? What was the problem, and how did you solve it?
In a recent project in the Middle East, the client had a large gas facility built using a combination of process technologies. These required the use of duplex stainless steel vessels, piping, and some stainless circuits due to the salt content and the resultant risk of chloride stress corrosion cracking (CSCC) from internal fluids. However, this facility is also coastal, which means a salty atmosphere subjects the pressure components to external chloride stress cracking (ECSCC) risks. Because of the higher temperatures of operation, the circuits were thermally insulated. As standard practice and based on past experience, 2205-grade duplex materials were used on all equipment, which the team considered as risk mitigation for both CSCC and ECSCC.
To verify and optimize, I closely studied the process conditions for each piece of equipment. Upon examination, I noticed that one very tall (50') and large (90" diameter) vessel, as well as two air-cooled exchangers downstream in the last leg of the circuit, had no internal salts. This made these pieces of equipment free from the risk of internal fluid-induced chloride stress corrosion cracking. The materials specification philosophy stated the use of a 2205-grade duplex for these pieces of equipment to mitigate ECSCC. Because they operate at hot temperatures above 200°F, which makes them still vulnerable to ECSCC, an additional layer of protection was applied by coating them with Thermal Spray Aluminum (TSA) coating. Duplex SS alone is 3-5 times more expensive than carbon steel, and adding TSA coating makes it even more expensive, adding an additional 20% cost due to the large surface areas requiring coating.
With my expertise in corrosion under insulation (CUI), materials, and in-depth knowledge of relevant standards, I sought alternative economical ways to address the ECSCC concern. Carbon steel is known to be resistant to ECSCC and, therefore, would be a good material to use. However, the internal fluids are still highly corrosive to carbon steel and would require protection from better metallurgy. I confirmed from process simulations and past experience that the salt content in this equipment is below the threshold of any risk for stainless steel. Using this combination, I suggested the use of CS with stainless steel clad vessel walls, externally coated using a marine offshore compatible 3-coat system. This avoids the use of both duplex and TSA, which dramatically reduced the cost by $220,000. For the large air coolers, the use of carbon steel was not viable due to thin tubes that can't be made of carbon steel and the tube-tube sheet joints that must be of the same material. I resolved this by removing thermal insulation on all three pieces of equipment since they were meant to cool down anyway, thereby allowing the surfaces to dry out and minimizing salt accumulation. This approach allows for the use of the more economical 316 stainless steel and then adding TSA to headers that operate at higher temperatures but have smaller surface areas, making them not very expensive to coat. The tubes are protected by the use of aluminum sleeves. This arrangement reduced the risk of ECSCC and also provided huge cost savings of $180,000.

This incorporation of CUI know-how, insulation knowledge, and materials expertise led to great savings, de-risking the possibility of cracking. These benefits will spill over into future projects in similar environments, compounding the savings further.
What are some of the most effective state-of-the-art engineering technologies that you've used to mitigate corrosion? How do these technologies work?
Corrosion requires the presence of an anode (an easily corroding metal), a cathode (noble metal), an electrically conductive fluid, and a metallic pathway. This often translates to the presence of water, acidic conditions, and/or oxygen. Removing one of these components can eliminate or minimize corrosion activity to acceptable levels. Over the years, I have employed a variety of corrosion mitigation measures in the oil and gas industry, such as:
Environment modifiers that change the corrosivity of the environment:
- Corrosion inhibitors that interfere with and retard corrosion processes,
- Drying by removing water from the system using dehydrators such as glycols
- Oxygen scavenging that reduces oxygen in the system to insignificant levels pH adjustment that reduces acidity to low corrosive levels
- Biocides that kill corrosion-causing bacterial populations such as chlorination
- Flow modification reduced velocities that eliminate erosion-corrosion, pressure & temperature management to avoid cavitation
- Desalination by removing salts, rendering fluids non-conductive
Barrier protection that prevents water and/or oxygen from contacting underlying metal:
- Liners – Rubber linings, plastic liners, tapes or wraps, CRA liners that separate corrosive fluids from metal
- Inorganic coatings – Such as polyurea, PTFE (Teflon), epoxy novolacs, ceramic coatings, mastics
- External covers – Sheets of plastic or metals that form weather barriers
Sacrificial protection that self-sacrifices offering protection for metal:
- Sacrificial anodes – by self-consuming and protecting metal, such as Zinc and Magnesium anodes
- Impressed current cathodic protection – a technique of driving electrons into the protected metal via the application of electric power
Barrier and Sacrificial combination:
- Coatings – polymers that form barriers to water and/or contain sacrificial particles that protect the metal, such as Epoxy coatings with Zinc epoxy primers
- Platings – similar to coatings but using metallic thin layers that either sacrificially corrode (hot dip galvanizing) or form a corrosion-resistant barrier (Ni-Co plating) or do both (Zn-Ni plating)
Design controls that eliminate water entrapment, such as crevices, stagnant spots, and under-insulation water drainage.
Materials selection that resists corrosion and cracking:
- Corrosion-resistant alloys – that are inherently resistant to corrosion, usually conferred by an invisible, adherent oxide layer, such as stainless steel with its chromium oxide layer
- Material processing – wherein heat treatments, cold working, and welding are all controlled to produce softer, tougher materials that resist particular forms of corrosion cracking, such as the normalization of carbon steels or low nickel welding fillers for sour services
- Surface engineering – recognizing that corrosion starts at the surface, these techniques modify the surface of metal to make a thin top layer corrosion-resistant
Over the years, I have used all of these techniques either in isolation or in combinations in novel ways to produce safe, reliable, and economical solutions that mitigate corrosion.
How do you determine which corrosion mitigation strategies and technologies to use for a particular building or facility? What factors do you consider?
The specific strategy and technology depend on the following:
- Particular corrosion forms expected
- External and internal environment parameters
- Cost
- Availability of materials
- Design lifetime
- Preference of user
- Phase of project
- Company experience
- Reliability
To balance a variety of these factors, I often employ a combination of techniques to solve a particular problem.
For instance, an existing contract with a client required the use of thermal spray aluminum coating on stainless steel piping to protect from chloride stress corrosion cracking. This eventually proved to be too difficult to apply due to cost and feasibility reasons at the fabricator's site. I utilized an economical alternative of wrapping the pipes with aluminum foil, supplementing it with hydrophobic insulation materials, and ensuring good drainage of insulation. All these measures combined reduced the risk of CSCC to low levels. The foil effectively works as both a barrier to water and also as a sacrificial anode that corrodes first before the pipe underneath. These solutions allowed the facility construction to progress without major schedule impacts.

In a different case, our company found itself dealing with recurring corrosion issues using carbon steel bolts on stainless steel flanges due to galvanic corrosion despite the use of Teflon coatings. These coatings, while providing lubricity, do not withstand abrasion and fail to provide adequate protection. If, instead, stainless steel bolts are used in marine environments, they could be subject to chloride stress corrosion cracking and galling issues over time, not to mention the increase in cost. Some proprietary bolt-coating technologies were initially standardized. They were corrosion-resistant but were made only in one geographic location, leading to high costs and lead times. To resolve this, I identified a combination of technologies that can address each property without compromise.

To provide protection from galvanic and atmospheric corrosion, I utilized a combination of zinc-nickel plating that forms barriers and corrodes sacrificially. I employed a specific composition of Zn to Ni content and sufficient thickness that resists rust for more than 3000 hours of ASTM B117 salt spray conditions. Then, to retain lubricity, I investigated and chose the latest Teflon coating formulation embedded with corrosion inhibitor components and defined the application acceptance criteria. This combination is tested to show more than 4000 hours of salt spray resistance and effectively replaces the costly proprietary option.
I often had to build high-pressure filter pressure vessels that required large body flanges. They are essentially huge doors, and the size of the vessel diameter could be 48", 55".

These flanges have more than 25 heavy-duty studs with nuts that require special bolt tools, are extremely heavy (upwards of 6,000 lbs), and take 20-30 minutes at the least to open completely. One common way to address the issue is to use quick opening closures (QOC), which are special technology doors made for high-pressure vessels. These QOCs can be opened in less than 2 minutes using hands. However, when operating in a corrosive environment, the materials required can be stainless steel or nickel alloys instead of carbon steel, which makes the whole equipment very expensive. To reduce cost, I deploy clad steels where a 3mm thin corrosion-resistant alloy is applied to the carbon steel vessel. However, QOCs themselves cannot be clad due to difficult geometries and machined sealing surfaces. Hence, I buy QOC in higher alloy steel and perform dissimilar metal welding to the carbon steel clad shell. Often, due to the thickness of steel, a heat treatment becomes necessary, which is detrimental to the higher alloy properties. I resolved that issue by employing buttering of welds, which makes the use of alloy QOC possible without subjecting it to heat treatment. As this example makes clear, there are multiple hurdles to cross in arriving at an economical and particularly effective solution.
How do you ensure that corrosion mitigation strategies are effective and sustainable over the long term? What maintenance and monitoring activities are necessary?
Among the corrosion mitigation strategies discussed previously, only corrosion-resistant materials require minimum maintenance as they are inherently resistant to various forms of degradation. There are exceptions to this rule, too, as seen in the case of cracking concerns under salty environments combined with acidic services. Even here, employing the highest metallurgy possible can provide peace of mind but will be enormously capital-intensive to do so.
In most cases, economical options utilize a combination of the techniques discussed above, all of which require regular inspection and monitoring. Corrosion is a micro phenomenon that starts and progresses in an invisible manner until a macro feature emerges, at which point it's often too late to stop. In facilities filled with miles of piping and tons of equipment, it is nearly impossible to monitor all portions to detect corrosion in its early stages. Hence, a Risk-Based Inspection method (RBI) is a necessary strategy to invest resources and expenses at strategic locations and times. Various industry standards guide this process, such as API 580, offering a general framework; API 581, providing corrosion-specific RBI; and API 570, 574, and 572, offering specific guidance for pipelines and pressure vessels. Similarly, DNV-RP-G109 is an excellent guide to Corrosion Under Insulation management using the RBI methodology.
As such, it's necessary to distinguish between monitoring and inspection activities, which together form the basis of maintenance:
- Monitoring – A periodic or continuous assessment of measurable variables that can be correlated to the effectiveness of a corrosion control method.
- Inspection – A series of activities that occur at specific but long intervals to determine the condition of the system.
NACE 3T199 provides excellent guidance on various corrosion monitoring techniques. These techniques can be broadly categorized into direct and indirect. Direct techniques can be intrusive or non-intrusive, while indirect techniques can be online or offline. Direct techniques entail the measurement of a parameter that is directly affected by corrosion or erosion. Conversely, indirect techniques involve measuring a parameter that either influences or is influenced by corrosion or erosion.
Intrusive techniques in internal monitoring encompass measurements that necessitate access through the pipe or vessel wall. It's important to note that the term "intrusive" or "non-intrusive" does not define whether the probe extends into the process flow. Intrusive techniques often involve the use of probes or test specimens, such as flush probe designs. They encompass a wide variety of tools, such as physical measurements via mass loss coupons, extended analysis coupons, electrical resistance, and electrochemical measurements like Linear Polarization Resistance (LPR), Electrochemical Noise, and Electrochemical Spectroscopy. Non-intrusive techniques allow measurements of variables without opening any nozzle. These techniques include Magnetic Flux Leakage measurement, live RT testing, Acoustic Emission technique, and Guided Wave testing, among others.
Indirect techniques can be classified as either online or offline. In online methods, measurements are conducted without removing the monitoring device from the process. These include measuring water chemistry, conductivity, pH, flow velocity, hydrogen monitoring, etc. Conversely, offline methods entail the removal of a sample or specimen for subsequent analysis, such as corrosion product sampling, fluid sampling, and testing. Each technique has its domain of usefulness and is suitable for a particular environment. Many standards are available for each individual technique. Some examples include NACE RP0775 for corrosion coupons, ASTM G31 for immersion testing of coupons, and G96 for electrochemical methods.
As for inspection, visual, radiography, ultrasonic, eddy current, dye penetrant, PMI, and thermographic are among the common techniques that are deployed at strategically planned frequencies based on risk analysis. Probability of Failure (POF) estimations guide this risk assignment, and locations or circuits with high POF scores are given greater attention and inspection budgets. A simple example of this assessment is to classify a set of pipelines into Class 1, 2, or 3 based on the severity of risk. Class 1 risk entails the release of lethal or hazardous substances, while Class 2 involves the release of hydrocarbons that are flammable upon a continuous fire source, and Class 3 comprises the least risky fluids, such as water. Class 1 will undergo thickness measurements every 3 years, whereas Class 2 intervals can be as long as 10 years. Such distinction allows strategic and systematic allocation of resources, minimizing impacts to facilities or personnel.
Can you walk us through the process of designing and implementing a corrosion prevention and control plan for a large building or facility? What are the key steps in this process?
Oil and gas facilities are highly complex networks of metallic/non-metallic pressure containers in various forms and shapes, monitored and controlled 24/7 with sophisticated instrumentation. These containers house hazardous, flammable, and highly corrosive fluids. A typical facility is designed to last 20-30 years. Given that corrosion is a complex phenomenon, with numerous forms of material degradation occurring microscopically, the undertaking of a corrosion prevention and control plan is of paramount safety, cost, and environmental significance. This evaluation is carried out systematically for all components of the facility in a highly structured manner, with due consideration given to both internal and external environments. The end user of the facility is ultimately responsible for providing all the necessary information and performance criteria.
The process begins by defining these environments using key parameters such as fluid contents, pressure, temperature, flow conditions, external atmospheric corrosivity, and contaminants like mercury and hazardous component limits. These conditions might change over the lifetime of the plant. Therefore, multiple scenarios encompassing all the above variables should be clearly defined.
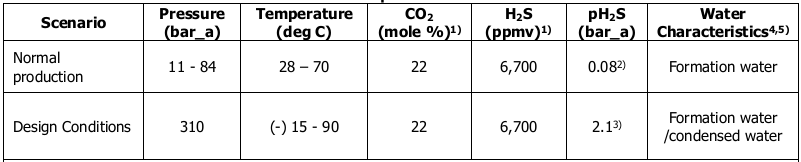
Apart from these, the end user must provide a basis for predictions, such as the required design life, accepted corrosion prediction models for various forms of corrosion, philosophy on inspection and maintenance practices, operational reliability requirements, and lessons learned from previous experiences. The process must also consider statutory and code limitations, as well as regulatory requirements. When providing this data, users should consider conducting actual corrosivity tests for the intended fluid compositions unless they have sufficient historical data to base assumptions on potential degradation mechanisms. A simple example of such identification is shown here.
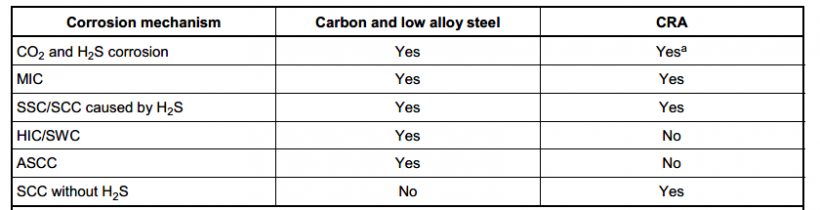
All of the above forms the design basis for material selection and corrosion mitigation plan. Often, the corrosion assessment is based on modified DeWaard Williams models, which may need to be supplemented by more sophisticated models depending on the level of confidence in the results. For example, a model that provides good predictions for long pipelines with CO2 corrosion may not yield accurate results for short variable sections of topside piping with high H2S.
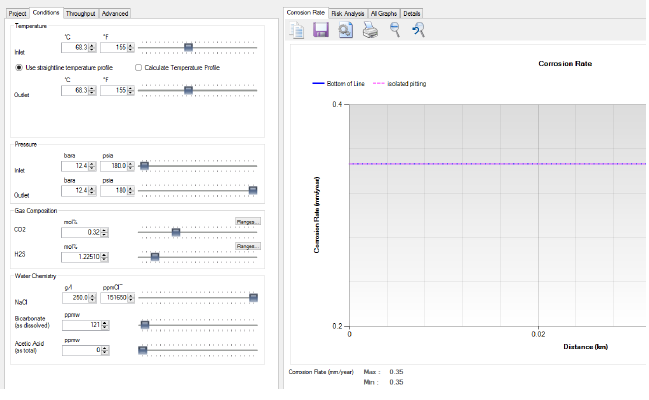
The results of this analysis are provided as a corrosion summary for all pipelines. Further, the final selections of suitable metallurgy based on multifactor influences are specified.
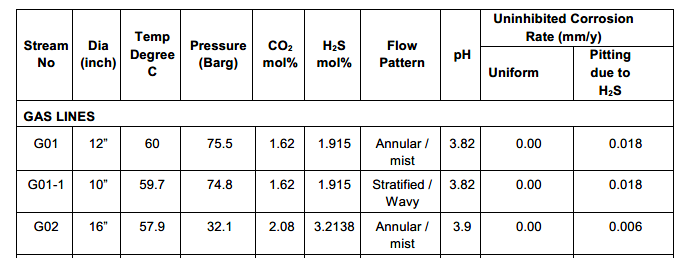
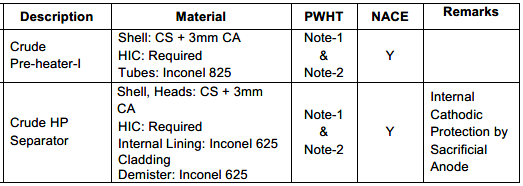
Finally, a Material Selection Report (MSR) is written to convey all the steps taken in the process that spell out the design basis, simulation entries, results, relevant standard guidelines, the final selections, and their justification. Often, an MSD, a Material Selection Diagram, is also generated, which looks like a version of the Process Flow Diagram that shows lines and equipment material details along with corrosion allowances and notes on degradation preventive measures like additions of corrosion inhibitors.
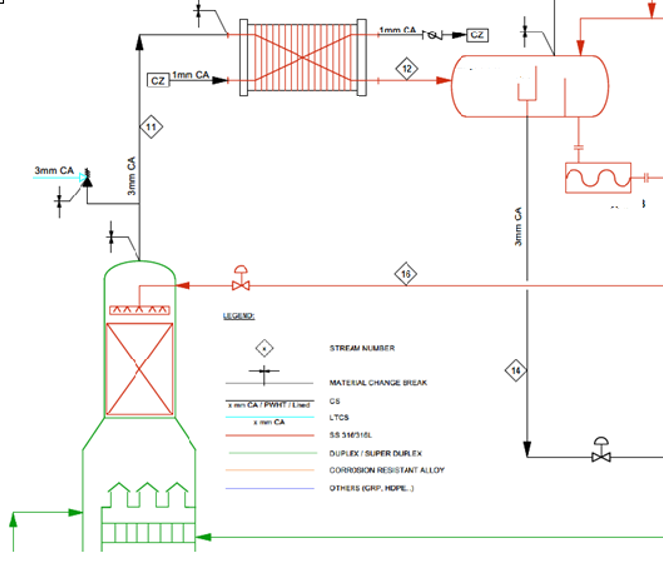
These reports should reflect materials of construction, design corrosion allowances, velocity limits, coatings and linings used internally or externally, sacrificial anode designs, injection of chemicals where necessary, and any special material processing considerations such as PWHT, stress relief, etc. Such details are broken down for every process-wet component, like valve trims, instrument diaphragms, and bolting washers.
How do you work with other stakeholders, such as maintenance personnel or building owners, to ensure that corrosion mitigation strategies are implemented effectively?
Corrosion management of an oil and gas facility is part of the life cycle asset management process. Due to the complexity of asset management in hazardous environments, it is critical to follow organizational procedures, safety protocols, systematic monitoring and inspection, and last but not least, communication. Many owners implement corrosion management strategies that define the operational and maintenance activities to prevent corrosion. Corrosion monitoring device data is used to simulate corrosion models and predict available life. Inspections shed further light on the actual status of the components.
The specifics of management techniques depend on the strategy selected during facility construction and its expected performance. Risk-based inspection and monitoring programs help in the judicious use of resources. Oil and gas facilities are increasingly digitized, which makes the facility safer for personnel through remote management while also allowing for more sophisticated electrochemical monitoring techniques that provide early signs of possible failures. The data collected can also be sent to external corrosion experts for deeper insights, to refine the monitoring and inspection programs, and to remediate any issues before they fail.
Can you describe any challenges you've faced in implementing corrosion mitigation strategies, and how did you overcome them?
For the design of an early production facility, a project encompassing 600 pieces of equipment worth over $100 million with a production capacity of 180 MMSCFD (Million standard cubic feet) of gas, the initial material selection shown on documents was a carryover from an old, similar facility.

Based on my extensive knowledge of process technologies, packing experience, and corrosion expertise, I found that the initial materials selected would result in significant design changes as they were economical and, if used, would require reinforcement from better material choices owing to severe risks of H2S. I demonstrated to the management that not only was the material selection improperly specified, but it also listed unsuitable coating systems, the absence of corrosion inhibitors, and inaccurate interpretations of industry codes/standards. With 10% CO2 and significant ppm levels of H2S (a lethal gas) that makes the systems highly corrosive, I proposed the use of corrosion inhibitors combined with risk mitigation testing requirements for sour service resistance. This replaced the previous choice of insufficient 6mm corrosion allowance and unreliable epoxy lining, leading to detrimental outcomes. In providing a complete solution, I extensively researched different compositions and corrosion inhibitor products with suppliers and conducted a comprehensive corrosion analysis for all upset and operating conditions of streams, accounting for possible sources of corrosion influencing species such as the formation of water carbonates, chlorides, CO2, and H2S.

In another large facility with a number of dissimilar metallic flange connection joints servicing corrosive fluids, the client had no specification for handling potential galvanic corrosion issues. Halfway into the project, I noticed the engineering team working on it had used regular spiral-wound metallic gaskets in most places and occasionally specified a company-standard legacy practice of phenolic gaskets. This facility had numerous variations in service fluid contents, temperature, and pressure. Upon examining the properties of the intended gasket polymer, I saw it neither met the chemical resistance nor the pressure or temperature resistance in the majority of the cases. In order to avoid the recurring nature of this problem, I undertook an extensive investigation of gasket materials and alternative electrical isolation techniques that prevent galvanic coupling. Like a general corrosion assessment, I identified hundreds of galvanic coupling joints and their individual service parameters, which ran into more than 15 categories. In order to simplify the problem, I worked in reverse, wherein I selected a few widely available, broadly corrosion-resistant gasket polymers and mapped them into these categories. Using this analysis, I defined three classes of services—severe, high temperature, and standard into which I could fit every joint. Each class had a corresponding definition of gaskets and kits using G10, G11 laminates, and Mica washers. Such assignment with the corresponding full definition of gasket kits allowed a simple MTO with replaceability, reduced cost, and eventual standardization.
Lastly, how do you stay up-to-date with the latest advances and trends in corrosion mitigation technologies, and how do you apply these to your work?
I have been a member of NACE (National Association of Corrosion Engineers, now renamed AMPP), the premier organization for corrosion expertise, for the past seven years. I am an active participant in conferences such as the AMPP annual conference and the Offshore Technology Conference every year. Through this journey, I regularly correspond with renowned corrosion & material experts and have immensely benefited from those conversations. In addition to all the above, I regularly take training for niche topics that complement my work, such as practical interpretation of microstructures in alloy steels, failure analysis methodologies, and corrosion modeling using advanced techniques. Apart from these, I read extensively on materials selection and corrosion mitigation subjects. This varies from ASM handbooks, Corrosion handbooks, and periodicals such as Corrosion Journal, ASME's Mechanical Engineering magazine, Coatings Pro, and Materials Performance magazine.
All these efforts improve my expertise and help sharpen the impact of my work. I use this expertise to train other engineers and steer them toward the right solutions. For example, I presented a training called "Misconceptions in Implementing NACE MR0175 in Oil and Gas Processing Equipment," which addresses over or underspecifying sour service requirements. I have used the knowledge acquired through professional organizations and journals in many projects to minimize risks, and over time, I write standards that incorporate the distilled essence of industry experience and my own. A simple example is my use of Inert Multipolymeric Matrix coatings for high-temperature services that go through transient CUI temperature ranges. When I originally encountered a special situation a decade ago, there was no available coating system, and I implemented IMPM coatings for a National Oil company with good results. Over the years, 3rd generation IMPM coatings have gained acceptance and are now shown in many user specifications.
* This is a contributed article and this content does not necessarily represent the views of sciencetimes.com














