As the world continues to struggle due to the rising COVID-19 cases, researchers worldwide are developing more than 155 vaccines against the coronavirus, with 22 of them are in human trails already.
Typically, it requires years of research and testing before vaccines reach the clinic, but scientists are racing to develop a safe and effective vaccine by next year.
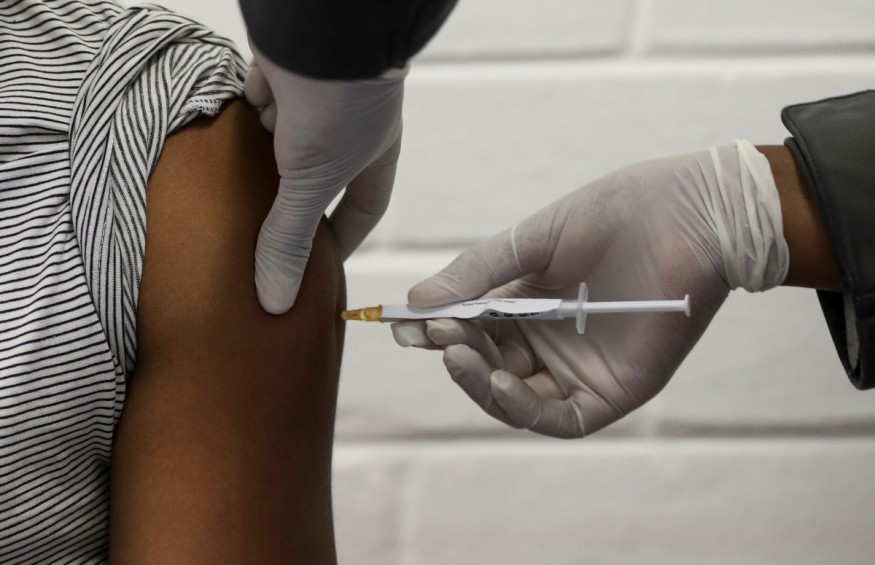
Scientists began deciphering the SARS-CoV-2 genome in January, and the first vaccine safety trials began in March. However, the road ahead is still uncertain as some trials will fail, and others may end without any definite result. A few of them may succeed in stimulating the human immune system to produce antibodies that will effectively kill the virus.
Russia 'first nation' to finish human trials of Coronavirus vaccine
Russian news agency TASS reports that Russia has become the first country to complete human trials of the COVID-19 vaccine on Sunday, July 12. More so, the news outlet added that the results have proven the effectiveness of the medication.
Human trials have been completed at the Sechenov University, and the subjects of the study will soon be discharged, said chief researcher Elena Smolyarchuk who heads the Center for Clinical Research on Medications at the university.
"The research has been completed and it proved that the vaccine is safe. The volunteers will be discharged on July 15 and July 20," said Smolyarchuk in an interview with TASS.
However, there was no further information yet on when their vaccine would be commercially reproduced. On June 18, Russia had allowed clinical trials of two forms of a potential COVID-19 vaccine developed by the Gamaleya National Research Center for Epidemiology and Microbiology.
Moreover, Burdenko Military Hospital received the first vaccine in the form of a solution for intramuscular administration. Then another vaccine in the form of a powder for preparing the solution for an intramuscular administration was carried out at the Sechenov First Moscow State Medical University.
The first stage of the Sechenov University vaccine research involved 18 volunteers in one group and a second group with 20 volunteers. After the vaccination, the volunteers were expected to remain isolated in a hospital for 28 days.
Early results of the vaccine tests on a group of volunteers showed that they were developing antibodies to the coronavirus.
"The data obtained by the Gamalei National Research Center for Epidemiology and Microbiology, proves that volunteers of the first and second groups are forming an immune response after injections of the vaccine against the coronavirus," said the Russian Defense Ministry.
Vaccine testing and approval process
To date, Russia has reported 719, 449 cases, and 11,188 deaths. According to the World Health Organization (WHO), there are at least 12.7 million global COVID-19 cases, while deaths have increased to more than 564,000.
Vaccines undergo a series of stages before getting approval from each country's approving body.
When the vaccine research is on its preclinical testing, scientists have already given the vaccine to animals. Moreover, some have already reached the Phase I safety trials, where scientists give the vaccine candidate to a small number of people. They proceed, giving it to hundreds of people, split into groups as they enter the Phase II expanded trials.
If it passes, it proceeds to Phase III efficacy trials to determine if the vaccine candidate protects against the coronavirus before being approved by the regulators in each country. A vaccine may receive emergency use authorization before getting the approval.
© 2026 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.












