In October, the United States Food and Drug Administration granted regulatory approval to a product labeled as Vuity, the first-ever eye drops that treat presbyopia. This eye care product was developed by the firm Allergan.
As specified in a ScienceAlert report, the joke about needing to have "longer arms" when a person grows older and tries to read tiny print is typical enough.
The reason for such a need is a natural decline in the ability of the eyes to focus on nearby objects, or a condition called presbyopia, which is why many people end up reaching for their reading glasses at an older age. Now, this report said, for the first time, there could be one more solution on hand.
Now, such drops have been made available on prescription. Meaning, millions of people can already survive without reading glasses, at least some of the time.
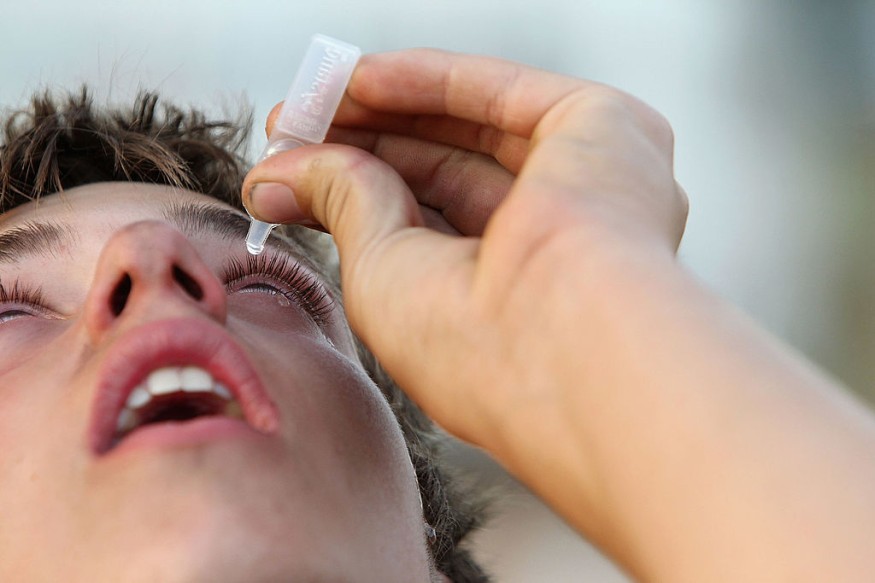
Presbyopia
In presbyopia, the eye's lens described in a Mayo Clinic report is getting harder and less elastic with age. More so, the muscles of the eyes are losing power over time. Therefore, it turns more difficult to concentrate on things that are close up, specifically when they are small.
Essentially, Selena McGee, an optometrist from the American Academy of Optometry, many Americans are dealing with presbyopia, which usually starts around age 40, by depending on reading glasses or resorting to workarounds such as zooming in on their electronic devices to see up close.
The eye drop then works, as it is formulated with 1.25 percent pilocarpine, a medication belonging to the class of miotics, the medicine that shrinks the pupil of the eye. Such drugs are used to lessen pressure in the eye, as well as in some diagnostic procedures.
Essentially, pilocarpine is shrinking the pupil; the eye is better able to focus, enhancing vision in the close range, while distance vision is ineffective.
Once an eye doctor has approved the treatment, the drops are described as "simple and safe" to apply. They can have an impact in as short as 15 minutes and can keep working for a maximum of six hours.
On the WHO's List of Essential List
Pilocarpine isn't without its risks. The drug has been around for a while and is on the World Health Organization's list of essential medicines, so we have a decent idea about its safety profile; adverse side effects include the very rare risk of retinal detachment, and it should also not be used if the person has inflammation of the iris.
In addition, since the eye drops are shrinking the pupils of an individual, they naturally impact the low-light vision of an individual; thus, there are warnings about driving at night or doing anything else hazardous in poor lighting.
Two-phase three clinical tests, yet to come out in a peer-reviewed journal, which involves more than 700 participants in all, were used to try Vuity compared to a placebo drop, with the therapeutic exhibiting a statistically substantial enhancement in vision among volunteers.
There were no serious adverse effects reported, although 14.9 percent of people reported they experienced headaches, and up to five percent of the volunteers reported other side effects like redness of the eyes, blurry vision, eye pain, irritation of the eyes, increased production of tears and eye irritation, a related report from The New York Times indicated.
Related information about Vuity eye drop is shown on Parkhurst NuVision's YouTube video below:
RELATED ARTICLE : Scientists Uncover Approach That Could Reverse Age-Related Vision Loss
Check out more news and information on Medicine & Health on Science Times.
© 2026 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.












