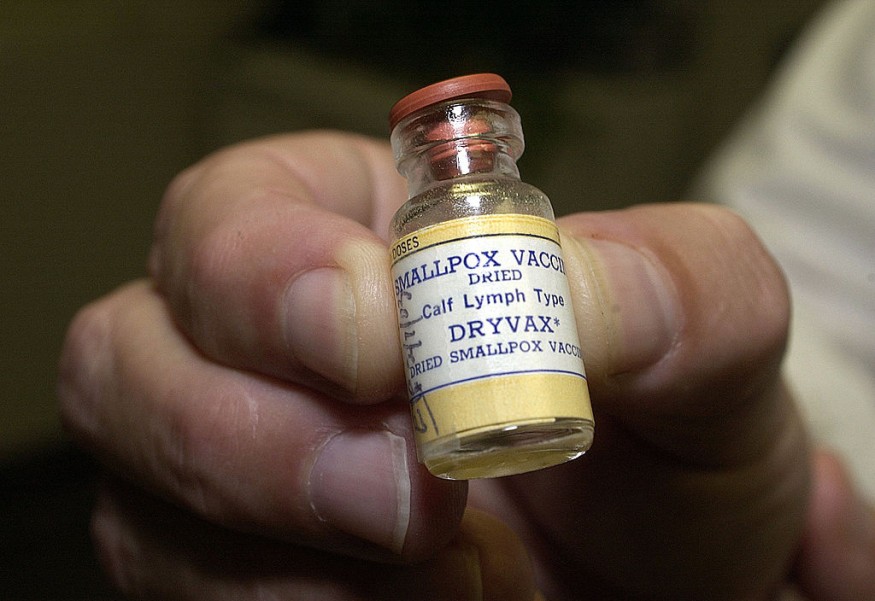
Bavarian Nordic A/S, a biotechnology company based in Denmark, announced that the US Biomedical Advanced Research and Development Authority (BARDA) has ordered the first doses of smallpox vaccines under a contract that will supply the freeze-dried version of the JYNNEOS® smallpox vaccine. The first dose could be manufactured and invoiced as early as next year.
The vaccine is known to provide protection not only against smallpox but also against monkeypox. Given the recent monkeypox outbreak in Europe and the US, will this prevent further transmission of the disease?
Ensuring Availability of Life-Saving Vaccines for All
The BARDA contract is expected to receive a total of approximately 13 million freeze-dried doses of the JYNNEOS smallpox vaccine that could be manufactured by 2023 and 2024, Precision Vaccinations reported. Although, most of the vaccines have already been manufactured.
Bavarian Nordic president and CEO Paul Chaplin announced in a press release that BARDA has exercised the first options under their contract to deliver the freeze-dried smallpox vaccines with an improved shelf-life manufactured at their facility. This is a significant milestone between Bavarian Nordic and the long-standing partnership with the US government to ensure the availability of vaccines for everyone.
JYNNEOS smallpox vaccine is composed of live, attenuated vaccinia virus that is incapable of replicating in the human cell but provides a potent immune response to protect people from smallpox and monkeypox.
Ongoing Monkeypox Outbreak in the US and Europe
There is currently a monkeypox outbreak in Europe, including the UK, Portugal, and Spain, with 36 suspected cases spread across the three countries, and one case in the US. Health authorities are still baffled about the origin of the monkeypox virus. There are some concerns that it may be spreading undetected through communities via a new route of transmission, NPR reported.
The UK Health Security Agency (UKHSA) chief medical adviser said in a statement on Monday, May 16, that this outbreak is rare and unusual since they have yet to identify its source and mode of transmission.
People usually catch monkeypox from animals in West Africa or Central Africa and import the virus to other countries via bodily fluids, like saliva or pus from the lesions. However, there is also evidence that it can be transmitted via sexual contact, which is a bizarre and new route to acquiring the infection.
They are now urging gay men and bisexual individuals to be aware of unusual rashes or lesions and immediately contact sexual health services if symptoms appear.
The Centers for Disease Control and Prevention (CDC) is watching the outbreak in Europe closely. In 2019, the US Food and Drug Administration (FDA) approved the JYNNEOS smallpox vaccine to prevent monkeypox outbreaks.
It was part of the Strategic National Stockpile (SNS) that aims to supply vaccines in case of potentially life-threatening diseases severe enough to cause supplies to be depleted. The vaccine was also approved in Europe in 2013. Below is a video of how freeze-dried vaccines are made and used:
RELATED ARTICLE: WHO Celebrates World Immunization Week 2022: See How Vaccines Protect People for More Than Two Centuries
Check out more news and information on Vaccines in Science Times.
© 2026 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.











