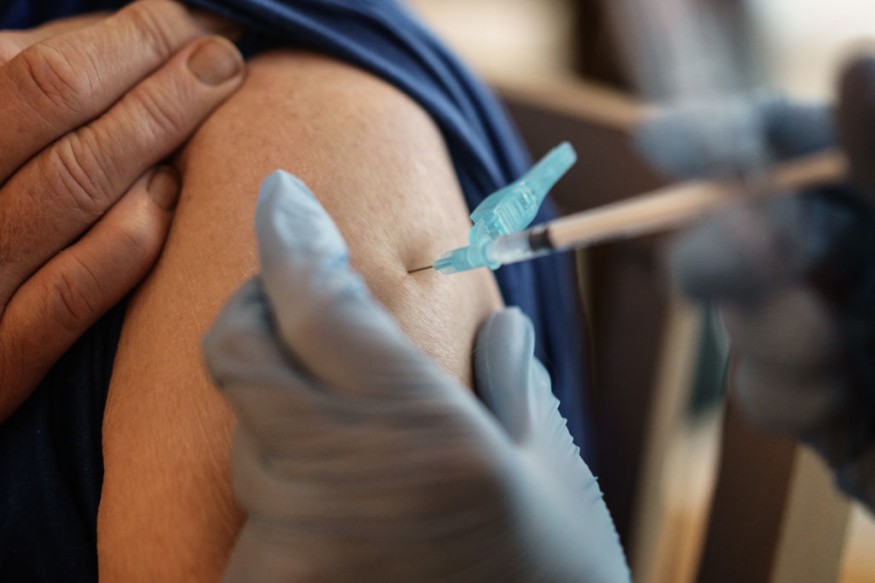
According to a new study from the Centers for Disease Control and Prevention, the rate of serious allergic reactions to the COVID-19 vaccine was higher but nevertheless "extremely rare" than with the flu shot.
Of about 1.9 million doses given, a total of 21 instances of anaphylaxis, none of them fatal, CDC researchers wrote in the Morbidity and Mortality Weekly Report on Wednesday. That averages out every 1 million doses to 11.1 instances, CDC officials told reporters.
Anaphylaxis is a significant allergic reaction that may be caused by food, medicine, bee stings and latex, as well as by a vaccine. If not managed quickly, the response may be lethal, usually with an epinephrine injection to expand the airways in the lungs.
Key Facts
The Vaccine Adverse Incident Monitoring System (VAERS), which the CDC and the Food and Drug Administration handles, keeps track of protection problems after a vaccine is made accessible to the public. They had obtained records of anaphylaxis other side effects of the Pfizer-BioNTech vaccine.
The COVID-19 vaccine from Pfizer-BioNTech was the first to obtain emergency use authorization from U.S. authorities. The first doses went into the arms of frontline healthcare staff on Dec. 14. The latest study by the CDC is focused on 1,893,360 doses issued by Dec. 23.
Those doses culminated in serious allergic reactions in 175 potential instances. Investigators investigating those incidents reported that 21 were anaphylaxis and 86 were other allergic reactions, respectively. There were no allergic responses at all in sixty-one cases, and seven are currently under investigation.
Of the 21 persons suffering from anaphylaxis, 17 have a history of allergies, including 7 individuals that have already had anaphylactic reactions.
Among the 21 patients, seventeen were examined in emergency departments, and four were committed to award. Intensive treatment was needed in three of those hospitalized patients.
By the time their reports were identified to the Vaccine Adverse Effect Monitoring Scheme, twenty of the patients had recuperated. Details of the 21st patient were not known. Still, the CDC researchers noticed that there were no records of fatalities connected to the Pfizer-BioNTech vaccine due to anaphylaxis.
The 21 patients varied from 27 to 60 years of age, with a median age of 40. Nineteen, or 90 percent, of them were women. The writers of the study noted that 64 percent were women in situations where the sex of a vaccine patient was identified. They also found out that after the 2009 flu pandemic, women were more prone to develop "immediate hypersensitivity" to the H1N1 influenza vaccine.
The quickest anaphylactic response occurred after only two minutes and the slowest occurred after 150 minutes after obtaining the COVID-19 vaccine. With 15 emerging within the first 15 minutes of the injection and three more developing between 15 and 30 minutes, the vast number of reactions arrived rapidly.
Of the patients, nineteen were administered with epinephrine.
The 21 cases were not concentrated in any particular geographic region and related to the vaccine's multi-lot doses.
More than four out of five incidents are deemed non-serious, unlike the other cases of allergic reactions. Rash or itchy eyes, itchy or scratchy neck, and minor respiratory symptoms were the most common recorded reactions to VAERS. Within 12 minutes after getting the vaccine, half of these reactions happened, and 90 percent of those who suffered from them were women.
Overall, within the first 10 days of the Pfizer-BioNTech vaccine launch, VAERS issued 4,393 records of adverse effects of some sort, according to the study. That is a 0.2 percent average.
What to Watch For
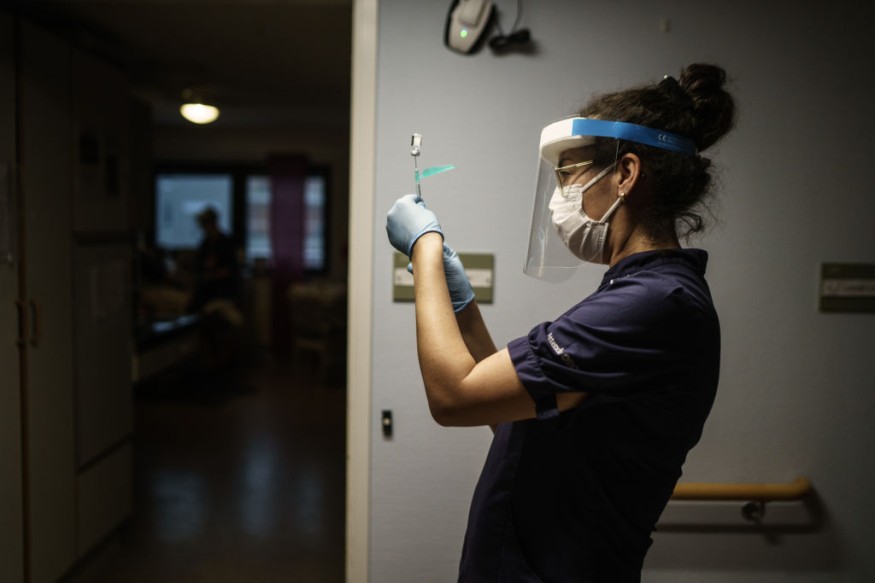
The CDC has also revised its vaccination recommendations and a related version established by Moderna, which was licensed for emergency use a week after the Pfizer-BioNTech medication was obtained.
The advice covers:
- Ensure the epinephrine is on hand at vaccine sites and available for usage.
- To classify people at high risk, question prospective vaccination candidates about their history of allergic reactions.
- Hold patients under surveillance for up to 30 minutes after getting the vaccine to rapidly handle instances of anaphylaxis.
- Ensure the healthcare professionals are qualified to understand the early symptoms of anaphylaxis by handing out the vaccine.
- Offer an epinephrine intramuscular injection instantly if anaphylaxis is suspected.
The first doses of the Moderna vaccine were given on Dec. 21, and over the 10-day duration of this research, less than 225,000 doses were administered. The CDC researchers said a separate paper on its side effects is in the works.
Check out more news and information on COVID-19 on Science Times.
© 2026 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.