Hearing aids will soon be available without a prescription, according to the Biden administration. The FDA has established a new category for over-the-counter hearing aids that do not require an examination or an expensive fitting. The devices could hit stores as early as mid-October.
FDA Approves Over-the-Counter Hearing Aids
Over-the-counter hearing aids have been in development for several years. Congress first passed legislation to allow over-the-counter sales in 2017, but the implementation of the regulations has been slow.
Last year, President Joe Biden issued an executive order directing the FDA to implement the rules as soon as possible, and the agency released a plan in the fall. The new final rule is the process's final step, which will go into effect in 60 days.
Health and Human Services Secretary Xavier Becerra said, "Today's action by the FDA represents a significant milestone in making hearing aids more cost-effective and accessible."
Headphone Companies May Start Selling Hearing Aids
For years, the distinction between headphones and hearing aids has been blurring. In 2018, Bose received the first FDA approval for a self-fitting hearing aid, which in some cases could be sold directly to consumers. Bose began selling hearing aids in 2021. The company halted the program and closed its healthcare division this spring. Because some state-level laws still restrict hearing aid sales to licensed hearing aid dispensers, the self-fitting hearing aid sold by Bose wasn't technically over the counter.
Jabra makes hearing aids, and its Enhance Plus wireless earbuds are marketed as hearing-enhancing. Apple is rumored to be considering entering the hearing aid market, while the AirPods Pro already has a Conversation Boost feature that improves voice recognition. Hearing aids are now supported by new Bluetooth audio standards.
However, with this new rule, hearing aids become yet another area where gadgets and medical devices collide, joining the ranks of bodily functions commoditized by tech companies, such as heart rate and blood oxygen levels.
It's unclear which headphone and tech companies, if any, will take advantage of this new hearing aid option. It will almost certainly lead to further hearing technology advancements from companies that manufacture hearing aids.
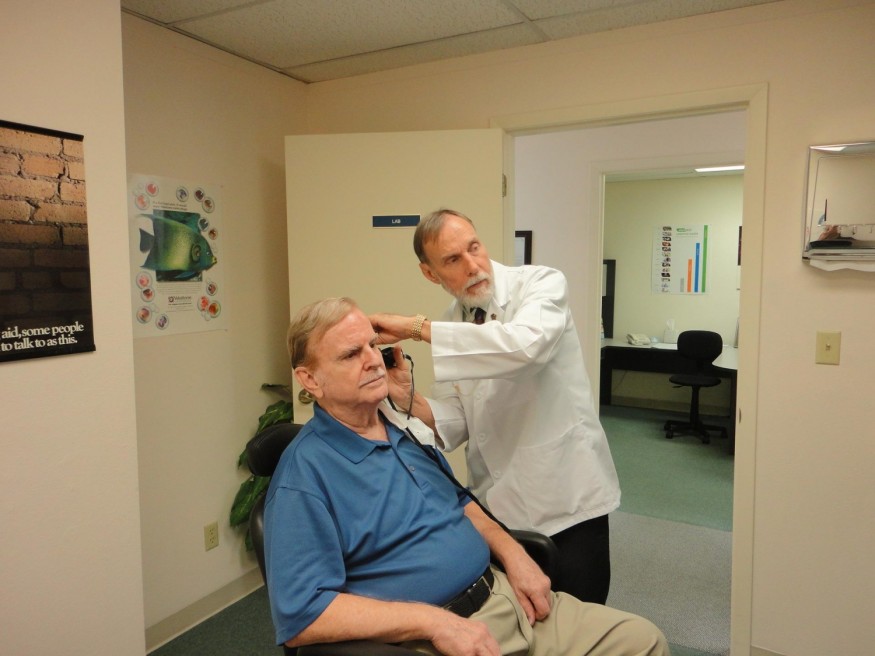
Audiologists Raised Concern on FDA Approved Over-the-Counter Hearing Aid
Audiologists, for their part, have expressed concern about over-the-counter hearing aids, stating that people with hearing loss still require a professional evaluation.
The Mayo Clinic shared on its website that a hearing evaluation is necessary to ensure that your audiologist can accurately diagnose your hearing loss and determine whether both ears are affected to the same extent.
A thorough diagnostic hearing evaluation can determine the extent of hearing damage and the underlying cause of hearing loss. Additional medical tests, such as blood tests or magnetic resonance imaging (MRI), may be ordered due to this type of evaluation. The purpose of these additional tests is to see if any underlying medical issues could be causing the hearing loss.
A hearing test can aid in diagnosing ear-related balance disorders such as Meniere's Disease, Positional Vertigo, Acoustic Neuromas, and Sudden Onset Hearing Loss. An earlier diagnosis can help you receive appropriate treatment right away.
RELATED ARTICLE : Researchers Design Hearing Aid Cheaper Than a Cup of Coffee
Check out more news and information on Technology in Science Times.
© 2026 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.











