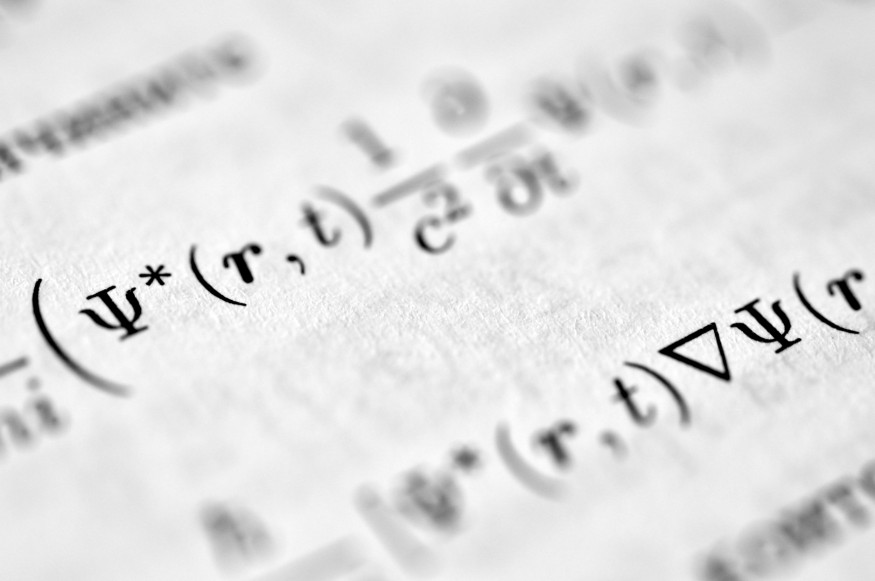Famous physics experiments like Young's double-slit and Rutherford's gold foil forced scientists to abandon deterministic models and embrace probabilistic and atomic frameworks. These experiments—from simple slits and pendulums to particle accelerators and atomic clocks—unlocked scientific breakthroughs that underpin electricity, nuclear power, and cosmology, transforming modern life. Technologies such as lasers, medical imaging, transistors, and GPS all rely on principles revealed by these precise measurements. Simple yet ingenious apparatus often revealed profound insights, proving that reality behaves far differently than everyday intuition might suggest.
These scientific breakthroughs demonstrate how experimental physics continually reshapes our understanding of the universe. By probing wave-particle duality, atomic structure, and relativistic effects, these studies not only expanded human knowledge but also created practical applications that influence daily life. From observing electron interference patterns to flying atomic clocks around the globe, these famous physics experiments illustrate the deep connections between theory, observation, and innovation.
8 Famous Physics Experiments That Changed Our Understanding of Reality
These eight famous physics experiments reshaped how scientists understand matter, light, and time. Each experiment challenged classical intuition and revealed fundamental principles of the universe. The results of these experiments paved the way for modern technology and scientific breakthroughs still in use today.
- Young's Double-Slit (1801): Light passing through two narrow slits created interference fringes, proving light's wave nature over Newton's particle theory. Later experiments with single electrons showed the same pattern, revealing the core of quantum mechanics and wave-particle duality.
- Michelson-Morley (1887): This interferometer measured light speed in perpendicular directions relative to Earth's orbit, producing a null result. The experiment disproved the luminiferous ether theory and laid the foundation for Einstein's special relativity.
- Rutherford Gold Foil (1909): Alpha particles fired at thin gold foil mostly passed through, but some scattered at sharp angles. This revealed a dense atomic nucleus at the center of mostly empty space, overturning the plum pudding model.
- Millikan Oil Drop (1909): Charged oil droplets suspended in an electric field allowed precise measurement of the electron's charge. The results quantified the fundamental unit of charge, e = 1.602 × 10⁻¹⁹ C, critical for atomic theory.
- Stern-Gerlach (1922): Silver atoms sent through an inhomogeneous magnetic field split into two discrete beams. This demonstrated quantized electron spin and introduced the concept of intrinsic angular momentum.
- Davisson-Germer (1927): Electrons diffracted off a nickel crystal produced interference patterns similar to light waves. This confirmed de Broglie's hypothesis of matter waves, supporting quantum mechanics.
- Photoelectric Effect (verified 1915): Light above a threshold frequency ejected electrons from a metal, independent of intensity. This experiment confirmed photon quantization and earned Einstein the Nobel Prize.
- Hafele-Keating (1971): Atomic clocks flown eastward and westward around Earth gained or lost time exactly as predicted by relativity. This provided real-world confirmation of time dilation due to velocity and gravity effects.
Famous Physics Experiments Revealing Wave-Particle Duality
Wave-particle duality became evident through famous physics experiments such as Young's double-slit and Davisson-Germer. These experiments demonstrated that light and matter can behave like waves while still arriving as discrete particles, defying classical physics. This discovery revealed that probability, rather than deterministic laws, governs subatomic behavior, challenging centuries of scientific assumptions.
The implications of wave-particle duality are immense. Technologies like electron microscopes rely on these principles to resolve structures at the nanoscale, including viruses and molecular complexes. Quantum tunneling, also a direct result of this duality, underpins flash memory and semiconductor devices, making it a foundational concept for everyday electronics like smartphones, laptops, and high-speed computing systems.
Scientific Breakthroughs Uncovering Atomic Structure
Experiments such as Rutherford's gold foil and Millikan's oil drop provided the first quantitative measurements of atoms' internal components. These famous physics experiments defined the atomic nucleus and quantified the electron charge, forming the basis for modern atomic theory. Understanding atomic structure enabled scientists to manipulate matter at a fundamental level, which later facilitated the development of transistors, semiconductors, and other key technologies.
Beyond electronics, atomic structure experiments paved the way for nuclear energy and medical applications. Fermi's Chicago Pile-1, the first controlled nuclear fission reactor, relied on insights from precise measurements of subatomic particles. Radiation-based medical technologies, including diagnostic imaging and cancer treatments, also trace back to these fundamental experiments, proving how foundational physics experiments translate into real-world innovations.
The Impact of Scientific Breakthroughs
Famous physics experiments continue shaping modern technology and scientific inquiry. Quantum computers exploit superposition observed in duality experiments, while gravitational wave detectors like LIGO rely on relativity validations. CERN's Higgs boson discovery and LIGO's black hole mergers extend our understanding of the universe's fundamental structure. By combining meticulous experimentation with theoretical insights, these breakthroughs illustrate how foundational physics experiments ripple across multiple disciplines, from computing and communication to energy and healthcare.
These experiments demonstrate that careful observation and measurement can uncover fundamental truths, influencing both scientific knowledge and everyday applications. The principles revealed in classical and modern physics experiments inform countless technologies, showing the interplay between experimental physics, theoretical models, and practical innovation. As new experiments continue, our understanding of reality will keep evolving, demonstrating the enduring power of scientific investigation.
Frequently Asked Questions
1. Why is Young's double-slit experiment so important?
Young's experiment demonstrated that light behaves as a wave, producing interference patterns. Later experiments with electrons revealed that particles can also display wave behavior. This showed the duality of matter, challenging classical mechanics. It laid the foundation for quantum mechanics and technologies like electron microscopes.
2. How did Rutherford's experiment change our understanding of atoms?
Rutherford's gold foil experiment revealed a dense atomic nucleus surrounded by electrons. It disproved the plum pudding model, reshaping atomic theory. This discovery allowed accurate predictions of chemical behavior. It also paved the way for nuclear energy and particle physics research.
3. What did the Michelson-Morley experiment prove?
The experiment measured light speed in different directions and found no difference, disproving the ether hypothesis. It provided evidence for Einstein's special relativity. This led to new understandings of space and time. GPS and satellite systems rely on these relativity principles for accuracy.
4. How does Hafele-Keating confirm relativity?
Atomic clocks flown around the Earth gained or lost nanoseconds consistent with relativity predictions. Velocity and gravitational effects caused measurable time dilation. The experiment confirmed Einstein's formulas in a practical, observable way. These findings are crucial for precise timing in GPS and telecommunications.
© 2026 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.