An enzyme called rubisco contains copper and is involved in the initial steps of carbon fixation. An ancient form of the enzyme was discovered by scientists which may be a missing link in the protein's evolution.
A new study by Lawrence Berkeley National Laboratory, UC Davis, and UC Berkeley was published in the journal Nature Plants describing the discovery of form 1 rubisco. The enzyme's origins gave insight into photosynthetic organisms within the plant food chain.
Doug Banda from Berkeley lab explained that rubisco is one of the oldest carbon-fixing enzymes on Earth. It is a "primary driver for producing food" by taking carbon dioxide from the atmosphere and converting it into sugar for photosynthetic organisms such as plants, cyanobacteria, and algae.
An Ancient Form of Rubisco
Form I rubisco is traced back to more than two billion years ago during the Great Oxygenation Event. At that time, oxygen accumulated in the atmosphere as cyanobacteria produced it through photosynthesis. As a result, anaerobic bacteria died and was leading up to the first mass extinction.
The roots of form I rubisco goes back to the time before cyanobacteria evolved. The enzyme's ancient form is also important in the timeline of the evolution of life. Studying rubisco may also help with the development of biofuels and sourcing renewable energy.
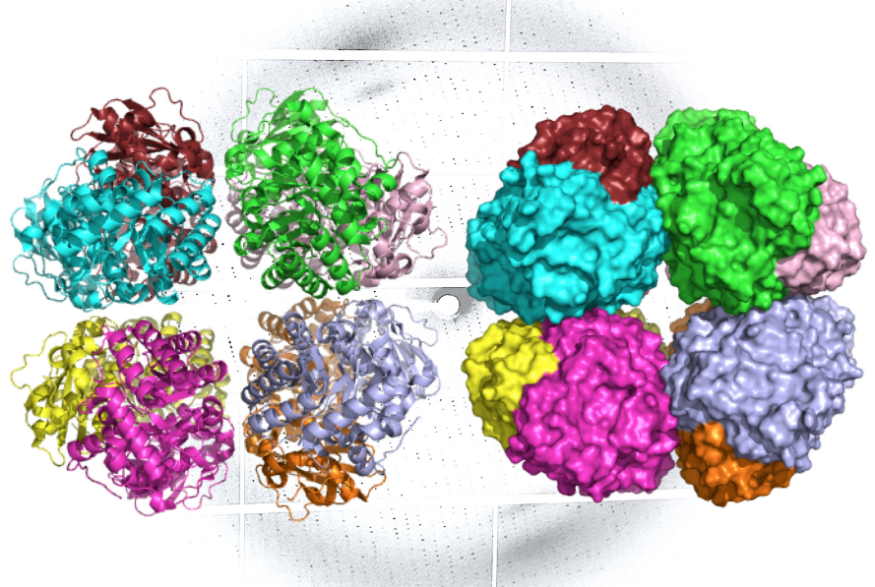
Rubisco form I was discovered after analyzing various groundwater samples. The genetic sequences of uncultured microorganisms expressed the enzyme's ancient form when the scientists used E. coli bacteria. Form I was then compared to other forms of rubisco. Structural biologists from Berkeley Lab created the enzyme's 3D model.
Using a method called X-ray crystallography, the team created images of the molecule with atomic-level resolution. The model also showed the structural changes of active rubisco form I. Another method called small-angle X-ray scattering was applied for the enzymes to appear as they naturally do in the environment.
Read Also: Unique Enzyme Combination Could Reduce Global Plastic Waste
Carbon Fixation and Photosynthesis
Unlike other forms of rubisco, form I contains protein subunits that enable the flow of information during protein synthesis as it combines with a larger subunit. Patrick Shih from UC Davis said that form I could have been what rubisco looked like and how carbon fixation occurred before the rise of oxygen by cyanobacteria billions of years ago.
Rubisco contains multiple protein domains that are bound with other molecules during photosynthesis. During the process, the molecule undergoes different arrangements in the various domains, explained Michal Hammel from Berkeley Lab. The combined technique displays the rubisco's natural behavior in real-world, physiological conditions, he said
The rubisco form lacking small subunits compared to other forms brings up questions about the evolution of life, shared Douglas Banda, such as the enzyme's functionality. In conclusion, the authors wrote that their study provides "insight into a key evolutionary transition of the most abundant enzyme on Earth and the predominant entry point for nearly all global organic carbon."
Jose Pereira shared that the team's collaboration for more than a decade has resulted in understanding problems in biology. The collaboration enables them to share different structural biological techniques to understand more about the evolution of life.
Check out more news and information on Enzymes in Science Times.
© 2026 ScienceTimes.com All rights reserved. Do not reproduce without permission. The window to the world of Science Times.












